|
| ||
Vol. 280, Issue 1, 138-145, 1997
Department of Pharmacology and Experimental Therapeutics, Loyola University of Chicago, Stritch School of Medicine, Maywood, Illinois
| |
Abstract |
|---|
The present study examines the consequences of prenatal fluoxetine exposure
on brain serotonin [5-hydroxytryptamine (5-HT)] neurons in male
offspring. Pregnant rats were administered either saline or
fluoxetine (10 mg/kg s.c.) daily from gestational day
13 through gestational day 20. The biochemical status of
brain 5-HT neurons was assessed in prepubescent and adult
offspring by measuring 1) the 5-HT and 5-hydroxyindoleacetic acid
content, 2) the density of [3H]paroxetine-labeled 5-HT
uptake sites and 3) the ability of the 5-HT-releasing drug
p-chloroamphetamine to reduce 5-HT content. Biochemical
parameters were assessed in the frontal cortex, hypothalamus,
hippocampus, striatum and midbrain. Comparative effects on dopamine
and norepinephrine content in selected regions were also determined.
Prenatal exposure to fluoxetine significantly reduced ( 28%) 5-HT content in the frontal cortex of
prepubescent but not adult male offspring. In contrast, in adult
progeny prenatal fluoxetine exposure produced a significant decrease
only in midbrain 5-HT content (
28%) 5-HT content in the frontal cortex of
prepubescent but not adult male offspring. In contrast, in adult
progeny prenatal fluoxetine exposure produced a significant decrease
only in midbrain 5-HT content ( 28%). In addition, p-chloroamphetamine markedly
reduced 5-HT content in all brain regions examined, but the ability
of p-chloroamphetamine to reduce 5-HT content was
significantly attenuated only in the midbrain of adult progeny
prenatally exposed to fluoxetine. No significant differences were
observed between control and fluoxetine-exposed progeny with respect
to brain 5-hydroxyindoleacetic acid content, the
5-hydroxyindoleacetic acid/5-HT ratio or the density of 5-HT uptake
sites, regardless of the brain region examined or the age of the
offspring. These data provide additional evidence that prenatal
exposure to fluoxetine can produce limited, rather than global,
changes in brain 5-HT neurons in male rat offspring and that the
effects observed are region-specific and age-dependent. The potential
functional consequences and clinical implications of these
alterations in brain 5-HT systems remain to be elucidated.
28%). In addition, p-chloroamphetamine markedly
reduced 5-HT content in all brain regions examined, but the ability
of p-chloroamphetamine to reduce 5-HT content was
significantly attenuated only in the midbrain of adult progeny
prenatally exposed to fluoxetine. No significant differences were
observed between control and fluoxetine-exposed progeny with respect
to brain 5-hydroxyindoleacetic acid content, the
5-hydroxyindoleacetic acid/5-HT ratio or the density of 5-HT uptake
sites, regardless of the brain region examined or the age of the
offspring. These data provide additional evidence that prenatal
exposure to fluoxetine can produce limited, rather than global,
changes in brain 5-HT neurons in male rat offspring and that the
effects observed are region-specific and age-dependent. The potential
functional consequences and clinical implications of these
alterations in brain 5-HT systems remain to be elucidated.
| |
Introduction |
|---|
Fluoxetine (Prozac) is a member of the class of antidepressants known as
selective serotonin reuptake inhibitors, because it preferentially
inhibits the transport of serotonin (5-HT) into presynaptic nerve
terminals and exhibits negligible affinity for a number of
neurotransmitter receptor subtypes (Peroutka and Snyder, 1980 ; Thomas et al., 1987
; Thomas et al., 1987 ; Fuller et al., 1991
; Fuller et al., 1991 ; Wong et al., 1991
; Wong et al., 1991 ). The high degree of selectivity of fluoxetine and its
minimal side effects, relative to the tricyclic antidepressants,
accounts, in part, for the widespread use of this drug. Consequently,
women of child-bearing age may constitute a large percentage of
the population of patients taking this medication, and the
therapeutic use of fluoxetine may continue throughout pregnancy.
). The high degree of selectivity of fluoxetine and its
minimal side effects, relative to the tricyclic antidepressants,
accounts, in part, for the widespread use of this drug. Consequently,
women of child-bearing age may constitute a large percentage of
the population of patients taking this medication, and the
therapeutic use of fluoxetine may continue throughout pregnancy.
Pohland et al. (1989) demonstrated that fluoxetine crosses the placenta and enters fetal
brain tissue, where it is likely to act at 5-HT transporters reported
to be present and functional in fetal brain (Mercado and Hernandez-R,
1992
demonstrated that fluoxetine crosses the placenta and enters fetal
brain tissue, where it is likely to act at 5-HT transporters reported
to be present and functional in fetal brain (Mercado and Hernandez-R,
1992 ; Ivgy-May et al., 1994
; Ivgy-May et al., 1994 ). Because 5-HT plays a critical role in the development of
5-HT neurons and target tissues in fetal brain (Lauder and Krebs,
1978
). Because 5-HT plays a critical role in the development of
5-HT neurons and target tissues in fetal brain (Lauder and Krebs,
1978 ; Chubakov et al., 1986
; Chubakov et al., 1986 ; Whitaker-Azmitia et al., 1987
; Whitaker-Azmitia et al., 1987 ; Azmitia and Whitaker-Azmitia, 1987
; Azmitia and Whitaker-Azmitia, 1987 ; Lauder, 1990
; Lauder, 1990 ), exposure of fetal brain to fluoxetine may affect the
regulation of fetal brain 5-HT and consequently the normal maturation
of brain 5-HT pathways. However, to date few studies have assessed
the neurochemical teratogenic potential of this drug. Studies in rats
and rabbits indicate that prenatal exposure to fluoxetine, at
moderate doses (i.e., doses that are not toxic to the mother),
does not produce gross physical abnormalities in the progeny, nor
does it affect fetal viability or litter size (Stanford and Patton,
1993
), exposure of fetal brain to fluoxetine may affect the
regulation of fetal brain 5-HT and consequently the normal maturation
of brain 5-HT pathways. However, to date few studies have assessed
the neurochemical teratogenic potential of this drug. Studies in rats
and rabbits indicate that prenatal exposure to fluoxetine, at
moderate doses (i.e., doses that are not toxic to the mother),
does not produce gross physical abnormalities in the progeny, nor
does it affect fetal viability or litter size (Stanford and Patton,
1993 ; Byrd and Markham, 1994
; Byrd and Markham, 1994 ; Cabrera and Battaglia, 1994
; Cabrera and Battaglia, 1994 ; Vorhees et al., 1994
; Vorhees et al., 1994 ), suggesting no physical teratogenic effects. Likewise,
evaluation using a variety of behavioral paradigms indicates that
prenatal fluoxetine exposure does not produce adverse effects in rat
offspring (Hoyt et al., 1989
), suggesting no physical teratogenic effects. Likewise,
evaluation using a variety of behavioral paradigms indicates that
prenatal fluoxetine exposure does not produce adverse effects in rat
offspring (Hoyt et al., 1989 ; Vorhees et al., 1994
; Vorhees et al., 1994 ). Few studies have attempted to investigate the
neurochemical teratogenic potential of fluoxetine with respect to
brain serotonin pathways.
). Few studies have attempted to investigate the
neurochemical teratogenic potential of fluoxetine with respect to
brain serotonin pathways.
Montero et al. (1990) demonstrated that prenatal exposure to fluoxetine decreased
[3H]imipramine binding to presynaptic 5-HT uptake sites in the
cortex of prepubescent rat offspring. Subsequently, Romero et
al. (1994)
demonstrated that prenatal exposure to fluoxetine decreased
[3H]imipramine binding to presynaptic 5-HT uptake sites in the
cortex of prepubescent rat offspring. Subsequently, Romero et
al. (1994) reported that prenatal exposure to fluoxetine reduced
5-HT receptor-stimulated phosphoinositide hydrolysis in the cortex of
prepubescent, but not adult, progeny. We previously reported that
prenatal exposure to fluoxetine reduced the density of
5-HT2A/2C receptors only in the hypothalamus of adult male
offspring (Cabrera and Battaglia, 1994
reported that prenatal exposure to fluoxetine reduced
5-HT receptor-stimulated phosphoinositide hydrolysis in the cortex of
prepubescent, but not adult, progeny. We previously reported that
prenatal exposure to fluoxetine reduced the density of
5-HT2A/2C receptors only in the hypothalamus of adult male
offspring (Cabrera and Battaglia, 1994 ). Consistent with the reduction in hypothalamic 5-HT
receptors, the neuroendocrine response to a 5-HT2A/2C
agonist was significantly attenuated in the fluoxetine-exposed
progeny, suggesting a functional consequence for the decrease in
postsynaptic receptors. Taken together, these studies indicate that
prenatal fluoxetine exposure can produce neurochemical and functional
alterations in pre- and postsynaptic components of brain 5-HT
pathways in rat progeny in the absence of visually apparent physical
terata.
). Consistent with the reduction in hypothalamic 5-HT
receptors, the neuroendocrine response to a 5-HT2A/2C
agonist was significantly attenuated in the fluoxetine-exposed
progeny, suggesting a functional consequence for the decrease in
postsynaptic receptors. Taken together, these studies indicate that
prenatal fluoxetine exposure can produce neurochemical and functional
alterations in pre- and postsynaptic components of brain 5-HT
pathways in rat progeny in the absence of visually apparent physical
terata.
The present study investigates the consequences of prenatal fluoxetine
exposure on the biochemical status of 5-HT neurons in various brain
regions, by measuring 1) the basal serotonin (5-HT) and 5-HIAA
content, 2) the density of [3H]paroxetine-labeled 5-HT uptake sites
and 3) the ability of the presynaptically acting, 5-HT-releasing drug
PCA to reduce regional 5-HT content, as previously reported (Fuller
et al., 1965 ; Fuller, 1980
; Fuller, 1980 , 1992
, 1992 ; Kuhn et al., 1985
; Kuhn et al., 1985 ; Adell et al., 1989
; Adell et al., 1989 ; Fattaccini et al., 1991
; Fattaccini et al., 1991 ). Comparative changes in DA and NE levels were also
determined in selected brain regions, as well as the changes in
markers of monoamine neurons that occur as a consequence of normal
maturation.
). Comparative changes in DA and NE levels were also
determined in selected brain regions, as well as the changes in
markers of monoamine neurons that occur as a consequence of normal
maturation.
Herein we report that prenatal fluoxetine exposure does not produce
comparable alterations in 5-HT neurons in all brain regions. The
reductions in basal 5-HT levels and the attenuated ability of PCA to
reduce 5-HT content in the present study appear to be region-specific
and age-dependent. These data are consistent with other findings,
from the limited reports available, indicating that prenatal
fluoxetine exposure can produce alterations in brain 5-HT neurons in
specific brain regions in rat offspring at specific postnatal ages.
Although it is presently unknown whether brain 5-HT neurons in human
offspring would be affected by prenatal exposure to fluoxetine,
comparable vulnerability of 5-HT systems in human offspring may be
clinically relevant, because dysfunction of 5-HT pathways has been
implicated in the etiology of various psychiatric disorders,
including depression, anxiety and aggressive behavior (Siever and
Trestman, 1993 ; Owens and Nemeroff, 1994
; Owens and Nemeroff, 1994 ; Baldwin and Rudge, 1995
; Baldwin and Rudge, 1995 ).
).
| |
Methods |
|---|
Animals. Pregnant Sprague-Dawley rats weighing 280 to 320 g were obtained from Zivic-Miller (Zelienople, PA) and maintained in a facility with controlled temperature (22-24°C), humidity (50-55%) and illumination (12/12-hr light/dark cycle, lights on at 7 A.M.). The determination of GD 0 was carried out by the supplier and was defined by the presence of a copulatory plug. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the National Institutes of Health.
Pregnant rats arrived in the laboratory on GD 5. Although we previously reported that our treatment paradigm does not alter maternal weight gain during pregnancy (Cabrera and Battaglia, 1994 ), all experimental animals were placed on a nutritionally
balanced liquid diet (Cabrera et al., 1993
), all experimental animals were placed on a nutritionally
balanced liquid diet (Cabrera et al., 1993 ), starting on GD 8 and continuing throughout the
injection period, to monitor daily nutritional intake. Experimental
dams received a single daily injection of either 0.9% saline
(2 ml/kg s.c.) or fluoxetine (10 mg/kg/2 ml s.c.), beginning on
GD 13 and ending on GD 20. After termination of the
injection paradigm, all animals had free access to food and water.
All offspring from each of the experimental groups were fostered to
untreated lactating dams, to eliminate the possible influence of
drug-induced differences in nurturing. All pups were weaned on PD
21. The number of samples (n) for each postnatal treatment
group was obtained by using a single pup from each of the litters.
Progeny were sacrificed by decapitation, as described below, at
PD 26 or PD 70. These ages represent pre- and postpubescent
time points, respectively.
), starting on GD 8 and continuing throughout the
injection period, to monitor daily nutritional intake. Experimental
dams received a single daily injection of either 0.9% saline
(2 ml/kg s.c.) or fluoxetine (10 mg/kg/2 ml s.c.), beginning on
GD 13 and ending on GD 20. After termination of the
injection paradigm, all animals had free access to food and water.
All offspring from each of the experimental groups were fostered to
untreated lactating dams, to eliminate the possible influence of
drug-induced differences in nurturing. All pups were weaned on PD
21. The number of samples (n) for each postnatal treatment
group was obtained by using a single pup from each of the litters.
Progeny were sacrificed by decapitation, as described below, at
PD 26 or PD 70. These ages represent pre- and postpubescent
time points, respectively.
Assessment of the functional status of serotonergic nerve terminals.
Offspring were sacrificed 1 hr after receiving a single injection of either
saline or the 5-HT releaser PCA (5 mg/kg i.p.). The brains were
quickly removed and placed on a cold petri dish, and the
hypothalamus, striatum, hippocampus, midbrain and frontal cortex were
dissected out. The brain regions were immediately placed in
cryovials, frozen in liquid nitrogen and stored at  70°C until used for HPLC analysis of monoamine content
or radioligand binding analysis of 5-HT uptake sites. Basal levels of
5-HT and its primary metabolite 5-HIAA were determined in each brain
region from animals receiving the saline injection before
sacrifice. As an index of 5-HT turnover, the ratio of 5-HIAA to 5-HT
values was determined for all acutely saline-challenged animals
from both prenatal treatment groups (Karstaedt et al., 1994
70°C until used for HPLC analysis of monoamine content
or radioligand binding analysis of 5-HT uptake sites. Basal levels of
5-HT and its primary metabolite 5-HIAA were determined in each brain
region from animals receiving the saline injection before
sacrifice. As an index of 5-HT turnover, the ratio of 5-HIAA to 5-HT
values was determined for all acutely saline-challenged animals
from both prenatal treatment groups (Karstaedt et al., 1994 ). For comparative purposes, DA and NE contents were
determined simultaneously with 5-HT and 5-HIAA levels.
). For comparative purposes, DA and NE contents were
determined simultaneously with 5-HT and 5-HIAA levels.
HPLC determination of biogenic amines. HPLC determination of brain
biogenic amines was carried out as described by Saller and Salama (1984) , with some modifications. Brain regions were sonicated
in 10 volumes of ice-cold 0.1 N perchloric acid containing
0.5 µM dihydroxybenzylamine as the internal standard used to
calculate the recovery of the biogenic amines. The homogenate was
then centrifuged at 20,000 × g for 15 min at 4°C.
Twenty-five to fifty-microliter aliquots of the supernatant were
injected into an HPLC system. The HPLC system consisted of a delivery
pump (model 501; Waters, Marlbourgh, MA) in conjunction with a
Waters 717 autosampler and an analytical column (Microsorb
C18, 5 µm, 150 mm × 4.6 mm;
Rainin, Woburn, MA) protected by a guard column (Microsorb
C18, 5 µm, 15 mm × 4.6 mm; Rainin). An
electrochemical detector (model LC-4C; Bioanalytical Systems, West
Lafayette, IN) with a glassy carbon electrode was used at a voltage
setting of +0.75 V vs. an Ag/AgCl reference electrode. The
mobile phase was composed of 9 g/liter monochloracetic acid,
0.25 mM EDTA, 0.375 g/liter 1-octanesulfonic acid and 1%
tetrahydrofuran (pH 3.0). The solvent flow was maintained at
2.0 ml/min. Standard solutions of 5-HT, 5-HIAA, NE and DA were
prepared in ice-cold 0.1 N perchloric acid containing
0.5 µM dihydroxybenzylamine. The system was run by Millennium
2010 Chromatography Manager, a computer program that performs
data acquisition, processing and management of chromatographic
information (Waters). Tissue precipitates were resuspended in
0.1 N NaOH to achieve a tissue concentration of approximately
30 mg/ml, and then 20-µl aliquots were taken for protein
determination according to the method of Lowry et al. (1951)
, with some modifications. Brain regions were sonicated
in 10 volumes of ice-cold 0.1 N perchloric acid containing
0.5 µM dihydroxybenzylamine as the internal standard used to
calculate the recovery of the biogenic amines. The homogenate was
then centrifuged at 20,000 × g for 15 min at 4°C.
Twenty-five to fifty-microliter aliquots of the supernatant were
injected into an HPLC system. The HPLC system consisted of a delivery
pump (model 501; Waters, Marlbourgh, MA) in conjunction with a
Waters 717 autosampler and an analytical column (Microsorb
C18, 5 µm, 150 mm × 4.6 mm;
Rainin, Woburn, MA) protected by a guard column (Microsorb
C18, 5 µm, 15 mm × 4.6 mm; Rainin). An
electrochemical detector (model LC-4C; Bioanalytical Systems, West
Lafayette, IN) with a glassy carbon electrode was used at a voltage
setting of +0.75 V vs. an Ag/AgCl reference electrode. The
mobile phase was composed of 9 g/liter monochloracetic acid,
0.25 mM EDTA, 0.375 g/liter 1-octanesulfonic acid and 1%
tetrahydrofuran (pH 3.0). The solvent flow was maintained at
2.0 ml/min. Standard solutions of 5-HT, 5-HIAA, NE and DA were
prepared in ice-cold 0.1 N perchloric acid containing
0.5 µM dihydroxybenzylamine. The system was run by Millennium
2010 Chromatography Manager, a computer program that performs
data acquisition, processing and management of chromatographic
information (Waters). Tissue precipitates were resuspended in
0.1 N NaOH to achieve a tissue concentration of approximately
30 mg/ml, and then 20-µl aliquots were taken for protein
determination according to the method of Lowry et al. (1951) . For each brain region, samples from prepubescent and
adult progeny were assayed simultaneously for brain monoamine
content.
. For each brain region, samples from prepubescent and
adult progeny were assayed simultaneously for brain monoamine
content.
Radioligand binding assay for 5-HT uptake sites. Regional 5-HT uptake
sites were measured in the cortex, hippocampus, striatum and midbrain according
to a previously published protocol (Battaglia et al., 1987 ), using a single saturating concentration of
radioligand. This method is sensitive to changes in the maximal
density of uptake sites. The density of hypothalamic 5-HT uptake
sites in fluoxetine-exposed offspring was previously reported
(Cabrera and Battaglia, 1994
), using a single saturating concentration of
radioligand. This method is sensitive to changes in the maximal
density of uptake sites. The density of hypothalamic 5-HT uptake
sites in fluoxetine-exposed offspring was previously reported
(Cabrera and Battaglia, 1994 ). Determination of the maximal density of 5-HT uptake
sites was carried out in a 5.0-ml assay containing 1 mg wet
weight of tissue and a single saturating (20 × Kd)
(Battaglia et al, 1987
). Determination of the maximal density of 5-HT uptake
sites was carried out in a 5.0-ml assay containing 1 mg wet
weight of tissue and a single saturating (20 × Kd)
(Battaglia et al, 1987 ) concentration (0.4 nM) of [3H]paroxetine
(20 Ci/mmol) in 50 mM Tris-HCl (pH 7.7, 25°C), 120 mM
NaCl, 5 mM KCl. Nonspecific binding was determined in the presence
of 1.0 µM citalopram. Tubes containing drugs and tissue were
incubated for 120 min at room temperature and then filtered
rapidly over Whatman GF/C filters that had been presoaked in 0.5%
polyethylenimine. The samples were then washed with 20 ml of
50 mM Tris-HCl (pH 7.7, 25°C). Filters were then added to
scintillation vials containing 5 ml of Ultima Gold (Packard
Instrument Co., Downers Grove, IL) scintillation fluid. The vials
were shaken for 60 min, and samples were counted for
2.5 min on a Beckman LS5000TD scintillation counter at an
efficiency of 60%. For each brain region, samples from prepubescent
and adult progeny were assayed simultaneously.
) concentration (0.4 nM) of [3H]paroxetine
(20 Ci/mmol) in 50 mM Tris-HCl (pH 7.7, 25°C), 120 mM
NaCl, 5 mM KCl. Nonspecific binding was determined in the presence
of 1.0 µM citalopram. Tubes containing drugs and tissue were
incubated for 120 min at room temperature and then filtered
rapidly over Whatman GF/C filters that had been presoaked in 0.5%
polyethylenimine. The samples were then washed with 20 ml of
50 mM Tris-HCl (pH 7.7, 25°C). Filters were then added to
scintillation vials containing 5 ml of Ultima Gold (Packard
Instrument Co., Downers Grove, IL) scintillation fluid. The vials
were shaken for 60 min, and samples were counted for
2.5 min on a Beckman LS5000TD scintillation counter at an
efficiency of 60%. For each brain region, samples from prepubescent
and adult progeny were assayed simultaneously.
Materials. NE hydrochloride, DA hydrochloride, serotonin creatinine sulfate and 5-HIAA free salt were obtained from Research Biochemicals International (Natick, MA). Monochloroacetic acid, 1-octanesulfonic acid sodium salt and tetrahydrofuran were obtained from J.T. Baker (Phillipsburg, NJ). [3H]Paroxetine was obtained from New England Nuclear (Boston, MA). Citalopram was provided by Lundbeck (Copenhagen, Denmark). Fluoxetine was generously provided by the Eli Lilly Co. (Indianapolis, IN). PCA and all other chemicals were obtained from Sigma Chemical Co. (St. Louis, MO).
Statistics. The data are represented as the group means and the S.E.M.
Statistical analysis of the data was performed by a two-way analysis
of variance. Individual group means were compared by Newman-Keuls
test (Steel and Torrie, 1960 ), using a computer program (SigmaStat; Jandel, San
Rafael, CA). P < .05 was chosen as the level of
significance.
), using a computer program (SigmaStat; Jandel, San
Rafael, CA). P < .05 was chosen as the level of
significance.
| |
Results |
|---|
Site-Specific Reductions in Monoamine Content in Fluoxetine-Exposed Offspring
5-HT and 5-HIAA. Table 1 reports
basal 5-HT levels in several brain regions in prepubescent and adult male
progeny prenatally exposed to either saline or fluoxetine. Prenatal
exposure to fluoxetine significantly reduced 5-HT content ( 28%) in frontal cortex only in prepubescent male
progeny. 5-HT content was not altered in either the hypothalamus,
hippocampus, striatum or midbrain in fluoxetine-exposed offspring
at PD 26. In contrast, in adult animals, basal 5-HT content was
significantly reduced only in the midbrain (
28%) in frontal cortex only in prepubescent male
progeny. 5-HT content was not altered in either the hypothalamus,
hippocampus, striatum or midbrain in fluoxetine-exposed offspring
at PD 26. In contrast, in adult animals, basal 5-HT content was
significantly reduced only in the midbrain (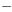 28%) of fluoxetine-exposed offspring (table 1). Basal
5-HT content in frontal cortex, hypothalamus, hippocampus and
striatum was comparable in control and fluoxetine-exposed adult
offspring. In contrast to the selective reductions in 5-HT content in
fluoxetine-exposed prepubescent and adult progeny, basal 5-HIAA
content was not altered by prenatal exposure to fluoxetine. As shown
in table 2, 5-HIAA
content in frontal cortex, hypothalamus, hippocampus, striatum and
midbrain was similar in control and fluoxetine-exposed progeny, at
both prepubescent and adult ages.
28%) of fluoxetine-exposed offspring (table 1). Basal
5-HT content in frontal cortex, hypothalamus, hippocampus and
striatum was comparable in control and fluoxetine-exposed adult
offspring. In contrast to the selective reductions in 5-HT content in
fluoxetine-exposed prepubescent and adult progeny, basal 5-HIAA
content was not altered by prenatal exposure to fluoxetine. As shown
in table 2, 5-HIAA
content in frontal cortex, hypothalamus, hippocampus, striatum and
midbrain was similar in control and fluoxetine-exposed progeny, at
both prepubescent and adult ages.
|
|
 ). As shown in table 3,
prenatal fluoxetine exposure did not produce any significant changes
in this index of serotonin turnover in any of the brain regions
examined (frontal cortex, hypothalamus, hippocampus, striatum
and midbrain), regardless of the age of the animal.
). As shown in table 3,
prenatal fluoxetine exposure did not produce any significant changes
in this index of serotonin turnover in any of the brain regions
examined (frontal cortex, hypothalamus, hippocampus, striatum
and midbrain), regardless of the age of the animal.
|
Catecholamines. Basal DA and NE levels were determined for comparative purposes and are shown in tables 4 and 5, respectively. Prenatal fluoxetine exposure did not alter basal DA levels in the hypothalamus, striatum or midbrain in either prepubescent or adult male offspring (table 4). The effects of prenatal fluoxetine exposure on DA levels in the frontal cortex and hippocampus could not be determined because the basal levels were below the detectable limit of our assay. As shown in table 5, basal NE content was not altered by prenatal fluoxetine exposure in either prepubescent or adult male offspring in any of the brain regions examined (frontal cortex, hypothalamus, hippocampus, striatum and midbrain).
|
|
Effect of Prenatal Fluoxetine Exposure on the Ability of a 5-HT-Releasing Drug to Reduce Regional 5-HT Content
A single injection of PCA resulted in significant (P < .05)
decreases in 5-HT content in all brain regions examined at both
postnatal times (fig. 1). The
magnitude of the reduction in 5-HT differed as a consequence of brain
region and postnatal age (20-67% reductions), with the greatest
reduction in 5-HT content being observed in the frontal cortex of
prepubescent male offspring (fig. 1). At PD
26, PCA administration significantly reduced hypothalamic 5-HT
content by 21% in saline-exposed progeny and by 34% in fluoxetine-exposed
offspring, in comparison with their respective basal 5-HT values
(table 1).
However, this 13% difference in the magnitude of reductions between
prenatal treatment groups did not reach statistical significance.
Similarly, PCA produced significant (P < .05) reductions in
5-HT content in the frontal cortex, hippocampus, striatum and
midbrain at PD 26 (fig. 1A), with
the magnitude of the reductions being comparable in control and
fluoxetine-exposed offspring. In contrast, in adult offspring, PCA
significantly reduced midbrain 5-HT content in both progeny groups,
but the magnitude of the reduction was significantly less in progeny
of fluoxetine-exposed dams (fig. 1B). In
contrast to the attenuated responses observed in midbrain, in other
brain regions (i.e., frontal cortex, hippocampus, striatum and
hypothalamus) significant but comparable PCA-induced reductions in
5-HT content were obtained in control and prenatal fluoxetine-exposed
adult offspring.
|
Effect of Prenatal Fluoxetine Exposure on the Density of 5-HT Uptake Sites
Prenatal exposure to fluoxetine did not alter the density of 5-HT uptake sites in prepubescent male progeny in either the frontal cortex, hippocampus, striatum or midbrain (table 6). Likewise, in adult progeny, no alterations in 5-HT uptake site density were observed as a consequence of prenatal fluoxetine exposure in any of the brain regions examined (table 6).
|
Age-Related Changes in the Functional Status of 5-HT Neurons in Control Progeny
Basal monoamine content. Several differences in basal monoamine content were observed as a consequence of normal maturation. For example, midbrain 5-HT levels were significantly greater in adult control progeny than in prepubescent control progeny (+61%) (table 1). In contrast, 5-HIAA content was significantly lower in adult control progeny, compared with values for prepubescent animals, in the hypothalamus, hippocampus, striatum and midbrain but not in the frontal cortex (table 2). These differential changes in 5-HT and 5-HIAA levels as a consequence of maturation resulted in significantly greater 5-HIAA/5-HT ratios (an index of 5-HT turnover) in adult control animals vs. their prepubescent counterparts, in all brain regions examined (table 3). With respect to age-dependent changes in catecholamines, basal DA levels were greater in striatum, but not in hypothalamus or midbrain, in adult control progeny (table 4). In contrast, NE levels in striatum were comparable at both postnatal ages. However, NE levels were significantly elevated in frontal cortex, hypothalamus, hippocampus and midbrain in adult offspring (table 5).
PCA-induced reduction of 5-HT content. In saline-exposed progeny, the ability of PCA to reduce 5-HT content was significantly greater in the frontal cortex of prepubescent offspring, in comparison with their adult counterparts (fig. 1). In contrast, PCA reduced 5-HT content in midbrain to a greater extent in adult than in prepubescent animals (fig. 1). However, reductions in 5-HT content in the hypothalamus, hippocampus and striatum after PCA administration were comparable between prepubescent and adult animals.
| |
Discussion |
|---|
The present study demonstrates reductions in brain 5-HT content only in
frontal cortex and midbrain in progeny after prenatal exposure to
fluoxetine. The reductions in 5-HT, in the absence of changes in the
number of 5-HT transporters in various brain regions, indicate that
this effect was not likely due to gross changes in 5-HT innervation
(Descarries et al., 1995 ). However, the attenuated response to a 5-HT releaser
in midbrain of adult progeny indicates possible changes in 5-HT
transporter function in this brain region produced by prenatal
exposure to fluoxetine. Taken together, these data indicate that
prenatal exposure to fluoxetine did not produce widespread or global
changes in 5-HT neurons. Rather, the effects of prenatal exposure to
fluoxetine on brain 5-HT systems were limited to selected brain
regions at specific developmental ages. Because only gross brain
regions were investigated in the present study, it is possible that
prenatal exposure to fluoxetine could have produced discrete changes
in 5-HT parameters in specific neuroanatomic loci that could not
be detected using homogenate binding assays. Consistent with this
possibility, autoradiographic data from our laboratory (Cabrera
et al., 1995
). However, the attenuated response to a 5-HT releaser
in midbrain of adult progeny indicates possible changes in 5-HT
transporter function in this brain region produced by prenatal
exposure to fluoxetine. Taken together, these data indicate that
prenatal exposure to fluoxetine did not produce widespread or global
changes in 5-HT neurons. Rather, the effects of prenatal exposure to
fluoxetine on brain 5-HT systems were limited to selected brain
regions at specific developmental ages. Because only gross brain
regions were investigated in the present study, it is possible that
prenatal exposure to fluoxetine could have produced discrete changes
in 5-HT parameters in specific neuroanatomic loci that could not
be detected using homogenate binding assays. Consistent with this
possibility, autoradiographic data from our laboratory (Cabrera
et al., 1995 ) have revealed a significant increase in 5-HT2A/2C
receptor density specifically in the entorhinal cortex of adult
fluoxetine-exposed offspring. However, changes in 5-HT2A/2C
receptors were not detectable when measured in cortical homogenates
of adult progeny prenatally exposed to fluoxetine (Cabrera and
Battaglia, 1994
) have revealed a significant increase in 5-HT2A/2C
receptor density specifically in the entorhinal cortex of adult
fluoxetine-exposed offspring. However, changes in 5-HT2A/2C
receptors were not detectable when measured in cortical homogenates
of adult progeny prenatally exposed to fluoxetine (Cabrera and
Battaglia, 1994 ).
).
Although the decreases in 5-HT content in adult frontal cortex represent
changes in 5-HT axons and terminals, it is unclear from the present
data whether the reduction of 5-HT in midbrain represents altered
5-HT in axons/terminals or perikarya. The reduction of 5-HT in
frontal cortex and midbrain could reflect a decrease in the synthesis
of 5-HT, because these changes occurred in the absence of any
alterations in 5-HIAA. It is interesting that the magnitude of the
reduction (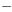 28%) in 5-HT was comparable in both brain regions
(i.e., frontal cortex and midbrain). However, it is not clear
from the present data why prenatal fluoxetine exposure produces
reductions in 5-HT only in frontal cortex and midbrain, which occur
at different postnatal developmental ages. In contrast to the changes
in 5-HT, prenatal exposure to fluoxetine did not alter basal DA or NE
levels in any of the brain regions examined, suggesting that
catecholamine neurons may be less sensitive to perturbation by
prenatal exposure to fluoxetine.
28%) in 5-HT was comparable in both brain regions
(i.e., frontal cortex and midbrain). However, it is not clear
from the present data why prenatal fluoxetine exposure produces
reductions in 5-HT only in frontal cortex and midbrain, which occur
at different postnatal developmental ages. In contrast to the changes
in 5-HT, prenatal exposure to fluoxetine did not alter basal DA or NE
levels in any of the brain regions examined, suggesting that
catecholamine neurons may be less sensitive to perturbation by
prenatal exposure to fluoxetine.
In prenatal fluoxetine-exposed progeny, another notable difference between
the frontal cortex and midbrain concerns the ability of the 5-HT
releaser PCA to reduce 5-HT content. In frontal cortex, PCA-induced
reductions in 5-HT were comparable in control and prenatal
fluoxetine-exposed progeny, regardless of developmental age. In
contrast, in midbrain of prenatal fluoxetine-exposed progeny, the
ability of PCA to reduce 5-HT was attenuated only in adult offspring,
at which developmental time the basal 5-HT was 28% lower than values
in control progeny. However, it is unlikely that the attenuated
response in midbrain is due specifically to the reduced 5-HT levels
in midbrain, because 5-HT was also significantly reduced by 28% in
frontal cortex, where there was no attenuation in the response to
PCA. One possibility is that 5-HT transporter function may be
differentially affected in frontal cortex vs. midbrain. PCA
enters 5-HT neurons via the 5-HT transporter and facilitates
the release of 5-HT (Kuhn et al., 1985 ; Rudnick and Wall, 1992
; Rudnick and Wall, 1992 ). Prenatal fluoxetine exposure may produce differential
effects on 5-HT uptake and/or release processes in 5-HT terminal
field regions vs. midbrain, a region containing both 5-HT
terminals and perikarya. Differences in the response to PCA could be
attributed to altered function of 5-HT transporters specifically on
midbrain perikarya. The present data, which demonstrate an attenuated
response to the 5-HT releaser PCA only in midbrain, are consistent
with an impairment in uptake and/or release processes in 5-HT
perikarya in fluoxetine-exposed adult progeny. Impaired entrance of
PCA into 5-HT neurons could be due to 1) changes in the density
of 5-HT uptake sites (either collectively or as the number of
sites/neuron) and/or 2) changes in the activity (i.e.,
Km) or maximal transport velocity (i.e.,
Vmax) of PCA for the 5-HT transporter. Likewise,
because PCA-mediated 5-HT release is mediated primarily via a
reversal of the 5-HT transport mechanism (Rudnick and Wall, 1992
). Prenatal fluoxetine exposure may produce differential
effects on 5-HT uptake and/or release processes in 5-HT terminal
field regions vs. midbrain, a region containing both 5-HT
terminals and perikarya. Differences in the response to PCA could be
attributed to altered function of 5-HT transporters specifically on
midbrain perikarya. The present data, which demonstrate an attenuated
response to the 5-HT releaser PCA only in midbrain, are consistent
with an impairment in uptake and/or release processes in 5-HT
perikarya in fluoxetine-exposed adult progeny. Impaired entrance of
PCA into 5-HT neurons could be due to 1) changes in the density
of 5-HT uptake sites (either collectively or as the number of
sites/neuron) and/or 2) changes in the activity (i.e.,
Km) or maximal transport velocity (i.e.,
Vmax) of PCA for the 5-HT transporter. Likewise,
because PCA-mediated 5-HT release is mediated primarily via a
reversal of the 5-HT transport mechanism (Rudnick and Wall, 1992 ), changes in 5-HT transporter kinetics
(Vmax and Km) and/or density
could affect the ability of PCA to reduce 5-HT. However, there
was no overall change in the density of 5-HT uptake sites in
homogenates of midbrain, or any other brain region, in
fluoxetine-exposed progeny. Therefore, the attenuated response to PCA
is unlikely to be the result of decreases in 5-HT transporter
density, unless such changes were restricted to 5-HT transporters
discretely localized on perikarya in dorsal and median raphe. Initial
in vitro autoradiographic data from our laboratory indicate no
changes in 5-HT transporter density in dorsal and median raphe
regions after prenatal exposure to fluoxetine (unpublished
observations). The absence of changes in the density of 5-HT uptake
sites, as reported herein, does not preclude the possibility that
prenatal exposure to fluoxetine may have affected the activity of
5-HT transporters in midbrain. Miller and Hoffmann (1994)
), changes in 5-HT transporter kinetics
(Vmax and Km) and/or density
could affect the ability of PCA to reduce 5-HT. However, there
was no overall change in the density of 5-HT uptake sites in
homogenates of midbrain, or any other brain region, in
fluoxetine-exposed progeny. Therefore, the attenuated response to PCA
is unlikely to be the result of decreases in 5-HT transporter
density, unless such changes were restricted to 5-HT transporters
discretely localized on perikarya in dorsal and median raphe. Initial
in vitro autoradiographic data from our laboratory indicate no
changes in 5-HT transporter density in dorsal and median raphe
regions after prenatal exposure to fluoxetine (unpublished
observations). The absence of changes in the density of 5-HT uptake
sites, as reported herein, does not preclude the possibility that
prenatal exposure to fluoxetine may have affected the activity of
5-HT transporters in midbrain. Miller and Hoffmann (1994) have shown that treatments that alter the activity of
the 5-HT transporter can do so independently of changes in the
density of 5-HT uptake sites.
have shown that treatments that alter the activity of
the 5-HT transporter can do so independently of changes in the
density of 5-HT uptake sites.
Another possible explanation for the attenuated PCA response in midbrain is
that prenatal exposure to fluoxetine may have altered the size of the
releasable pool of 5-HT. The majority of evidence suggests that PCA
releases 5-HT primarily from the cytoplasmic pool of 5-HT found
within 5-HT terminals (Sanders-Bush and Martin, 1982 ; Kuhn et al., 1985
; Kuhn et al., 1985 ; Adell et al., 1989
; Adell et al., 1989 ; Rudnick and Wall, 1992
; Rudnick and Wall, 1992 ). A reduction in the releasable pool of 5-HT may occur
independently of changes in the amount of 5-HT stored in secretory
vesicles. This would account for the differences noted in PCA effects
between frontal cortex and midbrain, despite the comparable
reductions in 5-HT content in the two brain regions. Because 5-HT
content and the response to PCA were both attenuated in midbrain of
adult progeny, it is possible that prenatal exposure to fluoxetine
could have altered 5-HT transporter function as well as 5-HT
synthesis and/or storage.
). A reduction in the releasable pool of 5-HT may occur
independently of changes in the amount of 5-HT stored in secretory
vesicles. This would account for the differences noted in PCA effects
between frontal cortex and midbrain, despite the comparable
reductions in 5-HT content in the two brain regions. Because 5-HT
content and the response to PCA were both attenuated in midbrain of
adult progeny, it is possible that prenatal exposure to fluoxetine
could have altered 5-HT transporter function as well as 5-HT
synthesis and/or storage.
In contrast to the midbrain, PCA reduced 5-HT in frontal cortex to a comparable extent in control and fluoxetine-exposed progeny, at both prepubescent and adult ages. The most parsimonious explanation for these findings is that, in the frontal cortex, uptake and release processes and/or the cytoplasmic (releasable) pool of 5-HT may not be affected by prenatal exposure to fluoxetine, regardless of whether basal 5-HT content is reduced. The reduction of basal 5-HT content in frontal cortex may be due to a reduction in the amount of 5-HT stored in secretory vesicles. Similarly, in other brain regions devoid of changes in basal 5-HT (i.e., hypothalamus, hippocampus and striatum), the ability of PCA to reduce 5-HT content was not affected by prenatal exposure to fluoxetine. This suggests that neither the amount of cytoplasmic 5-HT nor the uptake or release processes in these brain regions were altered by prenatal fluoxetine exposure.
Relatively few studies have quantitatively examined age-dependent changes in
monoamine content. With respect to catecholamines, the data from the
present study are consistent with the reports by Giorgi et al.
(1987) and Schwabe et al. (1992)
and Schwabe et al. (1992) , who observed significantly greater striatal DA levels
in adult offspring, in comparison with prepubescent animals.
Similarly, the elevations in basal NE content in adult vs.
prepubescent animals, across several brain regions, are consistent
with a previous report (Nomura et al., 1976
, who observed significantly greater striatal DA levels
in adult offspring, in comparison with prepubescent animals.
Similarly, the elevations in basal NE content in adult vs.
prepubescent animals, across several brain regions, are consistent
with a previous report (Nomura et al., 1976 ). However, unlike the age-dependent changes in striatal
DA content, hypothalamic and midbrain DA levels were similar in
prepubescent and adult progeny. With respect to serotonergic markers,
age-dependent differences in basal 5-HIAA levels were observed
in the hypothalamus, hippocampus and midbrain, with values being
significantly lower in adults than in prepubescent progeny (table
2). These
data are consistent with a report by Schwabe et al. (1992)
). However, unlike the age-dependent changes in striatal
DA content, hypothalamic and midbrain DA levels were similar in
prepubescent and adult progeny. With respect to serotonergic markers,
age-dependent differences in basal 5-HIAA levels were observed
in the hypothalamus, hippocampus and midbrain, with values being
significantly lower in adults than in prepubescent progeny (table
2). These
data are consistent with a report by Schwabe et al. (1992) , who observed lower 5-HIAA, but not 5-HT, levels in the
striatum of adult offspring, in comparison with prepubescent progeny.
The present study also demonstrates a selective increase in midbrain
5-HT content with maturation, which is consistent with a previous
report in whole brain (Nomura et al., 1976
, who observed lower 5-HIAA, but not 5-HT, levels in the
striatum of adult offspring, in comparison with prepubescent progeny.
The present study also demonstrates a selective increase in midbrain
5-HT content with maturation, which is consistent with a previous
report in whole brain (Nomura et al., 1976 ). Despite these site-specific and age-dependent changes
in 5-HT and 5-HIAA, no significant age-dependent differences were
observed in 5-HT uptake sites in any of the gross brain regions
examined. However, in control progeny, age-dependent differences were
observed in the magnitude of the PCA-induced reductions in 5-HT in
frontal cortex and midbrain. To our knowledge, the present study
represents the first published report of age-dependent differences in
the ability of the serotonin releaser PCA to reduce 5-HT content in
various regions of rat brain and the consequences of prenatal
fluoxetine exposure on this response.
). Despite these site-specific and age-dependent changes
in 5-HT and 5-HIAA, no significant age-dependent differences were
observed in 5-HT uptake sites in any of the gross brain regions
examined. However, in control progeny, age-dependent differences were
observed in the magnitude of the PCA-induced reductions in 5-HT in
frontal cortex and midbrain. To our knowledge, the present study
represents the first published report of age-dependent differences in
the ability of the serotonin releaser PCA to reduce 5-HT content in
various regions of rat brain and the consequences of prenatal
fluoxetine exposure on this response.
In summary, the present study demonstrates reductions in rat progeny 5-HT
content, limited to frontal cortex and midbrain, after prenatal
exposure to fluoxetine. These data suggest age-dependent and
site-specific, rather than global, changes in serotonin neurons
produced by prenatal fluoxetine exposure. Whereas cortical 5-HT
content is reduced in prepubescent fluoxetine-exposed progeny
and returns to control values in adult offspring, the reduction
in midbrain 5-HT content becomes apparent only after maturation
of the offspring. Likewise, the ability of PCA to reduce midbrain
5-HT content is altered only in adult offspring of fluoxetine-exposed
dams. These data suggest that the consequences of prenatal fluoxetine
exposure on 5-HT neurons may be influenced by maturational processes,
as previously reported for 5-HT2A/2C receptors and
receptor-mediated responses (Cabrera and Battaglia, 1994 ). Although it is unknown whether prenatal exposure to
fluoxetine would affect the development of 5-HT pathways in human
offspring, region-specific and age-dependent reductions in brain 5-HT
content and 5-HT neuronal function in human offspring may be of
clinical significance, because dysfunction of 5-HT pathways has been
implicated in the etiology of various clinical disorders (Siever and
Trestman, 1993
). Although it is unknown whether prenatal exposure to
fluoxetine would affect the development of 5-HT pathways in human
offspring, region-specific and age-dependent reductions in brain 5-HT
content and 5-HT neuronal function in human offspring may be of
clinical significance, because dysfunction of 5-HT pathways has been
implicated in the etiology of various clinical disorders (Siever and
Trestman, 1993 ; Owens and Nemeroff, 1994
; Owens and Nemeroff, 1994 ; Baldwin and Rudge, 1995
; Baldwin and Rudge, 1995 ).
).
| |
Acknowledgments |
|---|
The authors thank Dr. Louis Van de Kar and Qian Li for assistance with the collection of postnatal samples.
| |
Footnotes |
|---|
Accepted for publication September 13, 1996.
Received for publication August 15, 1995.
1 This study was supported in part by National Institute on Drug Abuse DA07741, National Science Foundation Grant GER-9253875 and the Loyola University Potts Foundation. T.M.C.-V. is the recipient of a National Science Foundation Minority Graduate Fellowship (GER-9253875).
Send reprint requests to: George Battaglia, Ph.D., Department of Pharmacology, Loyola University of Chicago, Stritch School of Medicine, 2160 South First Ave., Maywood, IL 60153.
| |
Abbreviations |
|---|
DA, dopamine; GD, gestational day; 5-HIAA, 5-hydroxyindoleacetic acid; HPLC, high-performance liquid chromatography; 5-HT, 5-hydroxytryptamine; NE, norepinephrine; PCA, p-chloroamphetamine; PD, postnatal day.
| |
References |
|---|
This article has been cited by other articles:
 |
L. G. Costa, L. Steardo, and V. Cuomo Structural Effects and Neurofunctional Sequelae of Developmental Exposure to Psychotherapeutic Drugs: Experimental and Clinical Aspects Pharmacol. Rev., March 1, 2004; 56(1): 103 - 147. [Abstract] [Full Text] [PDF] |
||||
|
| |||||
.gif) |
T. F. OBERLANDER, R. E. GRUNAU, C. FITZGERALD, A.-L. ELLWOOD, S. MISRI, D. RURAK, and K. W. RIGGS Prolonged Prenatal Psychotropic Medication Exposure Alters Neonatal Acute Pain Response Pediatr. Res., April 1, 2002; 51(4): 443 - 453. [Abstract] [Full Text] [PDF] |
||||
|
| |||||
.gif) |
T. M. Cabrera-Vera and G. Battaglia Prenatal Exposure to Fluoxetine (Prozac) Produces Site-Specific and Age-Dependent Alterations in Brain Serotonin Transporters in Rat Progeny: Evidence from Autoradiographic Studies J. Pharmacol. Exp. Ther., September 1, 1998; 286(3): 1474 - 1481. [Abstract] [Full Text] |
||||
|
| |||||
.gif) |
D. Bengel, D. L. Murphy, A. M. Andrews, C. H. Wichems, D. Feltner, A. Heils, R. Mössner, H. Westphal, and K.-P. Lesch Altered Brain Serotonin Homeostasis and Locomotor Insensitivity to 3,4-Methylenedioxymethamphetamine ("Ecstasy") in Serotonin Transporter-Deficient Mice Mol. Pharmacol., April 1, 1998; 53(4): 649 - 655. [Abstract] [Full Text] |
||||
|
| |||||
| ||||||||||||||||||||||||||||||||||||||||||||